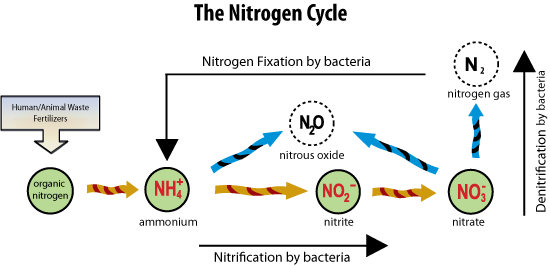
In particular, nitrogen is one of the nutrient pollutants that enter our water from runoff and groundwater. Nitrogen is available in the environment in multiple forms that are transformed within the nitrogen cycle. Nitrogen makes up 78% of our atmosphere and N2 is the gas form found in the air, called atmospheric nitrogen. N2 has to be fixed, or converted to ammonia (NH3) or ammonium (NH4+) by bacteria found in water, soil or plant roots. There are also manmade processes that convert N2 into NH3, which is used in fertilizer. In addition, some of this ammonia is converted into urea, a less soluble form of nitrogen, for use as a fertilizer.
Once in the soil, NH4 and NH3 can be converted by bacteria into nitrite (NO2-) and nitrate (NO3-). During this process, the byproduct N2O, the greenhouse gas nitrous oxide, escapes to the atmosphere. Nitrate is highly soluble form of nitrogen and it is readily leached into groundwater during rain events. If the groundwater is low in oxygen, then other bacteria can convert the nitrate into N2 gas, which is the process of denitrification.